
MindMed Shares Surge 10% As Psychedelics Gain Political Momentum Within Trump Admin
Shares of MindMed and other psychedelic names are bid this morning as psychedelic therapies are experiencing a major inflection point in U.S. policy and public awareness, with bipartisan political support, a surge of veteran advocacy, and rising investor interest—all despite significant regulatory headwinds.
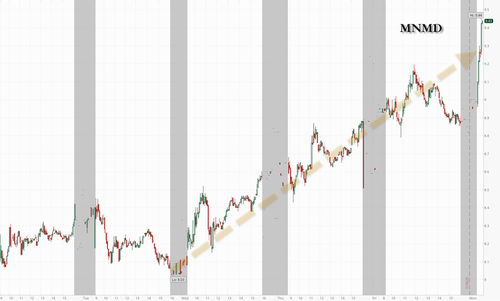
MindMed, one of our favorite psychedelic names, has been in a decisive uptrend since news first broke on July 16 that the Trump administration could be interested in fast-tracking the class of drugs.
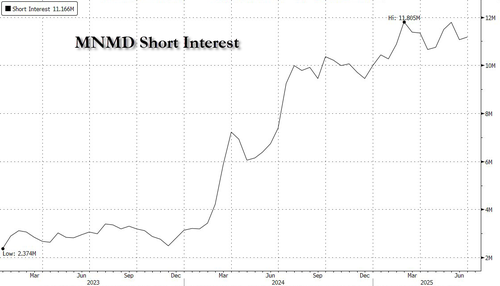
Short interest in MindMed has risen throughout 2024 and recently hit a 3 year high of nearly 12M shares.
Health Secretary Robert F. Kennedy Jr. recently signaled a dramatic shift in federal posture, telling lawmakers he expects psychedelic therapies for PTSD and depression to be available within 12 months.
“This line of therapeutics has tremendous advantage if given in a clinical setting and we are working very hard to make sure that happens within 12 months,” RFK Jr. said at the time.
His comments came as the FDA, now under Kennedy’s influence, weighs revisiting its recent rejection of MDMA (ecstasy) as a treatment for PTSD. Although an advisory panel voted against approval earlier this year—citing methodological flaws and safety concerns—the agency is reportedly considering reforms that could fast-track certain drugs, including psychedelics, by reducing trial burdens and review timelines.
The movement is getting unlikely support from conservative leaders, according to AP. Former Trump administration officials like VA Secretary Doug Collins and Texas Governor Rick Perry have joined a chorus of military veterans urging Washington to approve psychedelics.
Perry recently championed a $50 million research initiative in Texas to study ibogaine, a potent and controversial psychedelic, for opioid addiction and trauma.
AP writes that veterans remain at the heart of the push. Groups like Heroic Hearts Project and VETS (Veterans Exploring Treatment Solutions) have backed more than a thousand veterans seeking psychedelic-assisted therapy abroad due to current legal restrictions in the U.S. Stories of transformation—like those of Jon Lubecky and Casey Tylek, who credit MDMA therapy with saving their lives—continue to resonate across party lines and within Congress.
Despite the FDA’s resistance to MDMA approval under the Biden administration, the agency now appears more receptive under Kennedy’s leadership. Meanwhile, veteran-founded nonprofits, large-scale memorials, and Capitol Hill briefings are helping to build public pressure.
Still, concerns persist. Critics warn that weakening scientific standards for approval could undermine credibility and patient safety. Prominent researchers caution that political enthusiasm must not outpace rigorous evidence, especially given allegations of data manipulation and ethical lapses in some MAPS-affiliated studies.
Yet the momentum is undeniable. With Washington warming to psychedelic medicine and several states launching their own research programs, the psychedelic sector is seeing renewed investor optimism for the first time in years.
Tyler Durden
Mon, 07/21/2025 – 10:40

















